Abstract
Background: R-CHOP is a standard treatment for previously untreated DLBCL, however, more than one-third of patients are not cured by R-CHOP and there is a clear need for more effective novel first-line treatment combinations. Glofitamab is a CD20xCD3 T-cell-engaging bispecific monoclonal antibody with a novel 2:1 (CD20:CD3) configuration that confers high-avidity bivalent binding to CD20 on B cells (unlike the 1:1 format of conventional bispecific antibodies, which confers monovalent binding to CD20); this bivalency for CD20 enables combination with anti-CD20 antibodies, including rituximab. Glofitamab has shown high activity as a single agent in patients with heavily pretreated and/or highly refractory DLBCL (Dickinson et al. ASCO 2022).
Preliminary findings from the safety run-in phase of the ongoing NP40126 study (NCT03467373) demonstrated tolerable safety with glofitamab (Glofit) + R-CHOP as first-line therapy for patients with DLBCL (Ghosh et al. ASH 2021); here, we present efficacy and safety data from both the safety run-in portion and the expansion stage of this study.
Methods: Patients received R-CHOP in Cycle (C)1 with the aim of tumor debulking to mitigate cytokine release syndrome (CRS) risk. In total, 6-8 21-day cycles of R-CHOP were administered. Intravenous glofitamab was given during C2 (Day [D]8, 2.5mg; D15, 10mg) and at the target dose (30mg) from C3D8 onwards (21-day cycles). Hospitalization was at the discretion of the investigator for those patients enrolled in the expansion stage.
Response was assessed by PET-CT using Lugano criteria (Cheson et al. J Clin Oncol 2014). Patients who were scheduled to reach end of treatment (EOT) by the data cut-off were considered efficacy-evaluable (EOT population). CRS events were graded using ASTCT criteria (Lee et al. Biol Blood Marrow Transplant 2019). Other adverse events (AEs) were graded using CTCAE.
Results: At data cut-off (May 25, 2022), 56 patients were enrolled (safety population); of these, 46 had reached their scheduled EOT assessment (EOT population). Two patients in the EOT population were withdrawn from the study prior to receiving glofitamab (Grade [Gr] 5 infusion-related reaction [IRR] related to rituximab at C1D1; Gr 4 thrombosis following R-CHOP); the total number of patients exposed to glofitamab 30mg + R-CHOP was 44. Median age was 68 years (range: 21-84) and 54 pts (96.4%) had Ann Arbor Stage III/IV disease. In the EOT population, median IPI score was 3 (IPI 1: 2.2% [1/46], IPI 2: 30.4% [14/46], IPI 3: 34.8% [16/46], IPI 4: n= 28.3 [13/46], IPI 5: n= 4.3% [2/46]).
After a median 5.6 (range: 5.1-10.3) months follow up, the complete metabolic response rate was 76.1% (35/46) and the overall response rate was 93.5% (43/46) in the EOT population (BOR).
Of 56 patients in the safety population, Gr ≥3 AEs occurred in 40 (71.4%) patients and Gr ≥3 AEs related to glofitamab in 13 (23.2%) patients. Serious AEs (SAEs) were reported in 18 (32.1%) patients and SAEs related to glofitamab in five (8.9%) patients. Gr 5 AEs were reported in three (5.4%) patients (COVID-19 pneumonia, n=2; IRR to rituximab, n=1). AEs leading to dose modification/interruption of glofitamab occurred in 11 (19.6%) patients, including COVID-19 pneumonia (n=3) and COVID-19 (n=2). Median dose intensity was 100% for all R-CHOP components. One AE leading to discontinuation of glofitamab was reported (cerebrovascular accident, considered unrelated to glofitamab).
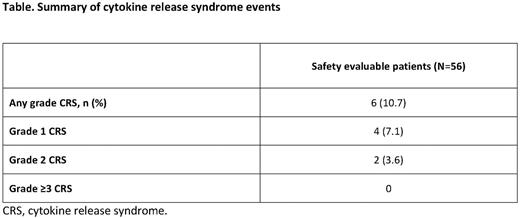
There were no severe Gr 3-5 CRS events and Gr 1-2 CRS was reported in six (10.7%) patients: Gr 1, n=4; Gr 2, n=2. All CRS events occurred during C2-3 and were resolved. Neurologic AEs (NAEs) occurred in 22 (39.3%) patients (of which the majority were Gr ≤2 [20/22]); Gr 3 NAEs included cerebrovascular accident (n=1) and herpes zoster (n=1). No glofitamab-related NAEs potentially consistent with immune effector cell-associated neurotoxicity syndrome were reported. Neutropenia was reported in 27 (48.2%) patients (Gr ≥3 neutropenia: Gr 3, n=6; Gr 4, n=19) and serious infection in nine (16.1%) patients.
Conclusions: The low incidence and severity of CRS, minimal toxicity and promising efficacy reported in this analysis suggest that glofitamab can be safely combined with R-CHOP as a fixed-duration treatment for patients with first-line DLBCL. The dose intensity of R-CHOP was maintained in all patients. Glofit + R-CHOP may be suitable for the outpatient setting.
Disclosures
Topp:KITE, BMS, Janssen, Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche, Regeneron, KITE, BMS: Research Funding; Universitätsklinikum Würzburg: Ended employment in the past 24 months; Universitätsklinikum Würzburg: Current Employment. Dickinson:Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD: Consultancy; Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD, Amgen: Honoraria; Roche, Novartis, Kite, Gilead, MSD, Takeda, Celgene: Research Funding. Ghosh:Gilead/Kite: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria; Loxo/Lilly: Consultancy, Honoraria; Incyte/Morphosys: Consultancy, Research Funding; TH Therapeutics: Consultancy, Honoraria, Research Funding; Epizyme: Speakers Bureau; AbbVie: Research Funding; Roche/Genentech: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen/Pharmacyclics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Beigene, Incyte, Karyopharm, Roche/Genentech, Novartis, Loxo Oncology, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie: Research Funding; Gilead, AstraZeneca, Bristol Myers Squibb, Phamacyclics, Janssen, Epizyme: Speakers Bureau. Santoro:Abb-vie: Speakers Bureau; Amgen: Speakers Bureau; Celgene: Speakers Bureau; Sanofi: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli-Lilly: Speakers Bureau; Sandoz: Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Incyte: Consultancy; Roche: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Pinto:Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Incyte (Italy), Merck Sharp and Dohme, Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Merck Sharp and Dohme, Incyte (Italy): Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bosch:Karyospharm: Honoraria; Astra Zeneca: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Fox:Roche: Other: Travel to scientific congress; Celgene/BMS, Gilead/Kite, Incyte, Janssen, Roche, Takeda: Speakers Bureau; BeiGene: Research Funding; Abbvie, AstraZeneca, Atarabio, Celgene/BMS, GenMab, Gilead/Kite, Incyte, Janssen, Morphosys, Ono, Roche, Takeda: Consultancy. López-Guillermo:Hospital Clinic de Barcelona: Current Employment; Roche, Kite/Gilead, Celgene, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding. Carlucci:Roche Holding AG: Current Employment, Current equity holder in publicly-traded company. Wu:Roche (China) Holding Ltd.: Current Employment; Roche: Current equity holder in publicly-traded company. Humphrey:Roche: Current equity holder in private company, Current holder of stock options in a privately-held company; Roche Products Ltd: Current Employment. Baumlin:Hoffmann La Roche: Current Employment. Barrett:Roche Products Ltd. UK: Current Employment, Current equity holder in private company. Qayum:Roche: Current Employment, Current equity holder in publicly-traded company. Morschhauser:Genentech: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific monoclonal antibody with a 2:1 (CD20:CD3) configuration that facilitates bivalent binding to CD20 on B cells, and monovalent binding to CD3 on T cells. Glofitamab redirects T cells to eliminate normal and malignant B cells. Glofitamab is an investigational agent. Rituximab (Rituxan) is a CD20-directed cytolytic antibody indicated for the treatment of adult patients (pts) with: relapsed or refractory, low grade or follicular, CD20-positive, B-cell NHL as a single agent; previously untreated follicular, CD20-positive, B-cell NHL in combination with first-line chemotherapy (chemo) and, in pts achieving a CR or PR to a rituximab product in combination with chemo, as single-agent maintenance therapy; non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line CVP chemo; previously untreated diffuse large B-cell, CD20-positive, NHL in combination with CHOP or other anthracycline-based chemo regimens; previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal